

Next-Generation TIL Therapy
Our next-generation technology platforms are designed to optimize
outcomes with TIL cell therapy
IOV-4001 is our lead genetically modified TIL program. It uses the pioneering gene-editing TALEN® technology licensed from Cellectis to inactivate the gene coding for the programmed cell death protein-1 (PD-1), and develop next-generation therapies.
IOV-4001: PD-1 Inactivated TIL Therapy
T cells, upon encountering cancer cells, produce PD-1, a checkpoint receptor that is activated by proteins (PD-L1 and PD-L2) found on cancer and other immune cells.1
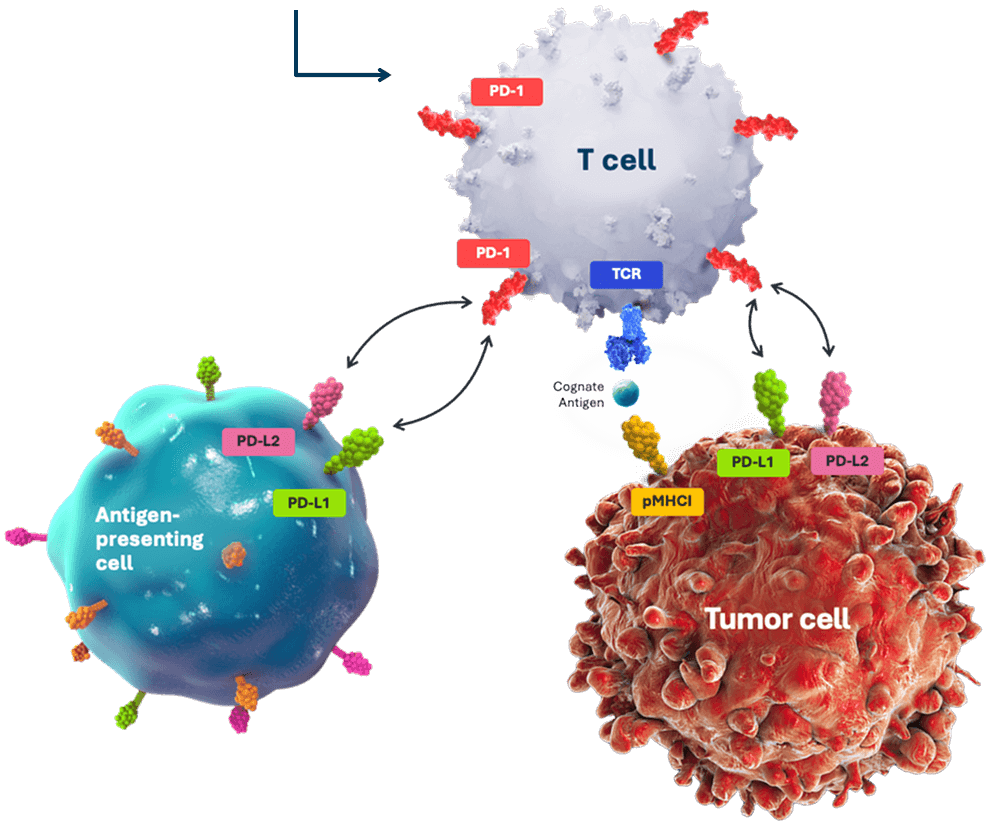
PD-1 is inactivated using TALEN, restoring the ability of TIL cells to kill cancer cells.2*
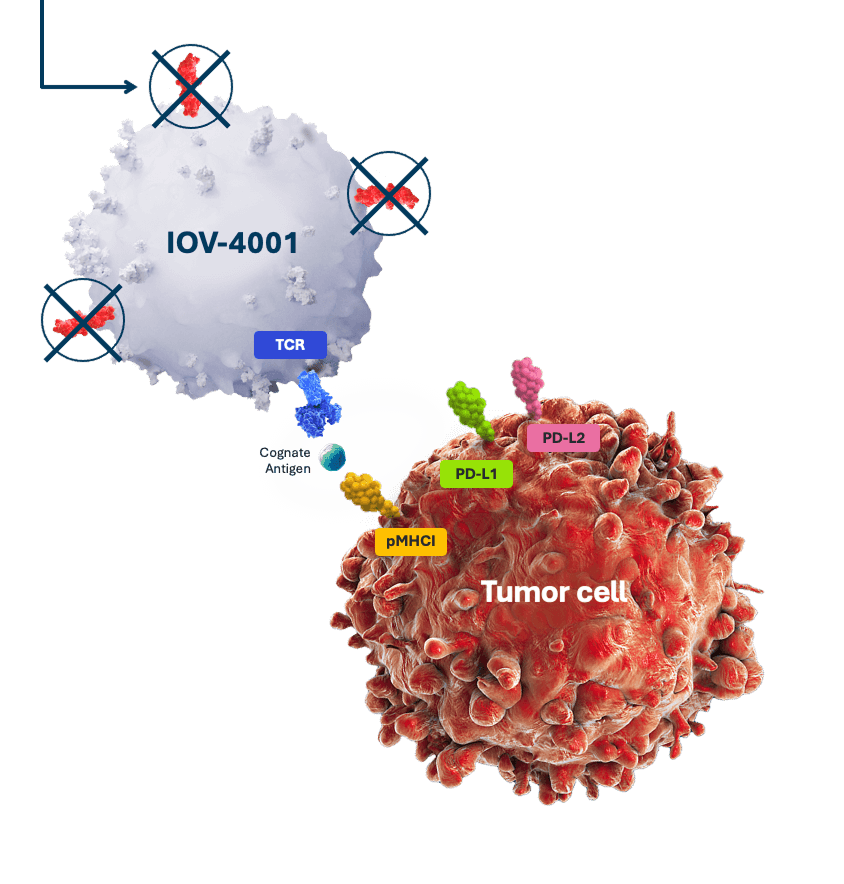
*Licensed from Cellectis.
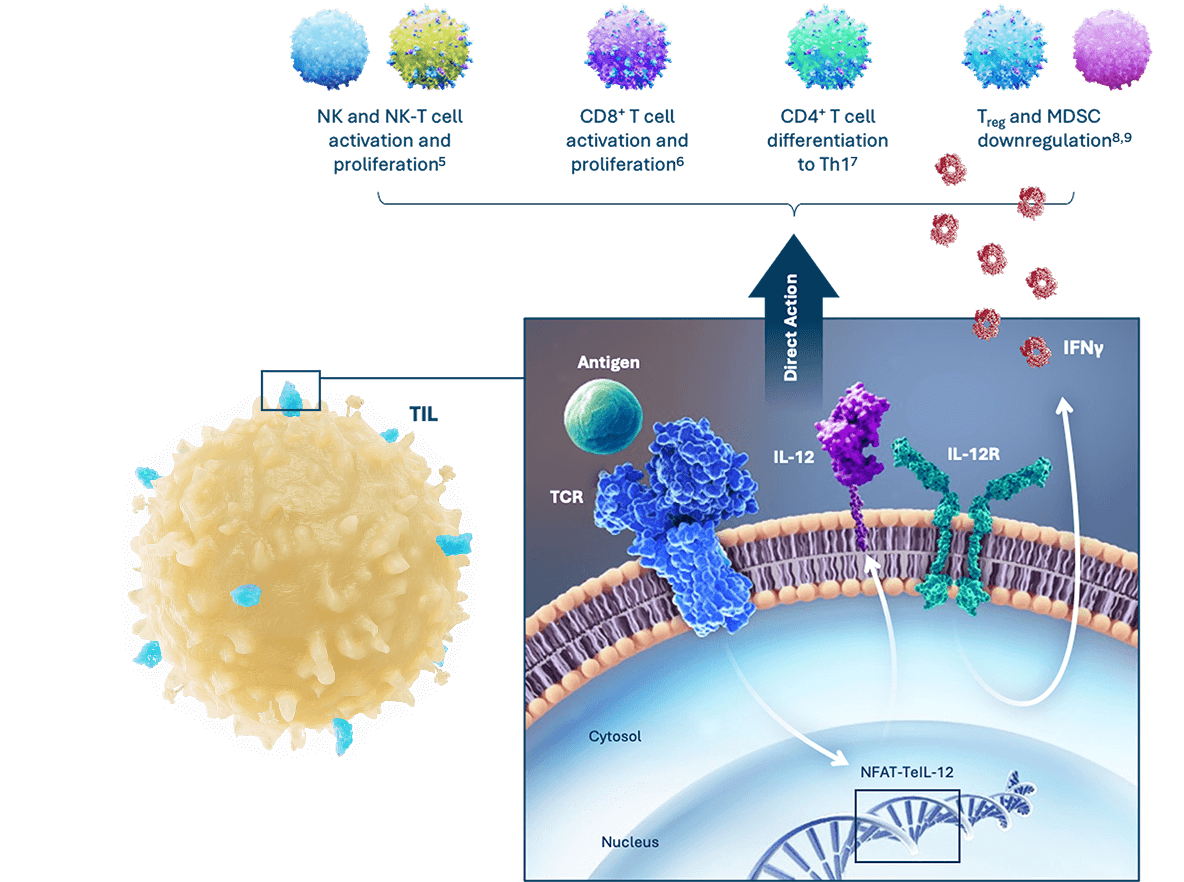
A genetically engineered, inducible, and tethered IL-12 TIL cell therapy, designated IOV-5001, is in investigational new drug-enabling studies.
IOV-5001: IL-12 TIL Therapy to Increase Efficacy
- Tethered IL-12 TIL cells can improve efficacy by remodeling the suppressive TME into an immuno-supportive state
- IL-12 shows independent clinical efficacy, with safe delivery to the TME being the primary challenge3,4
- Expression of IL-12 on IOV-5001 is induced upon antigen encounter in the TME3,4
- IOV-5001’s expressed IL-12 is tethered to the membrane surface of TIL to avoid release into circulation (shedding)4
- Inducible IL-12 expression in the TME and lack of IL-12 shedding are expected to allow increased IOV-5001 cell doses and improved TIL efficacy in solid tumor cancers
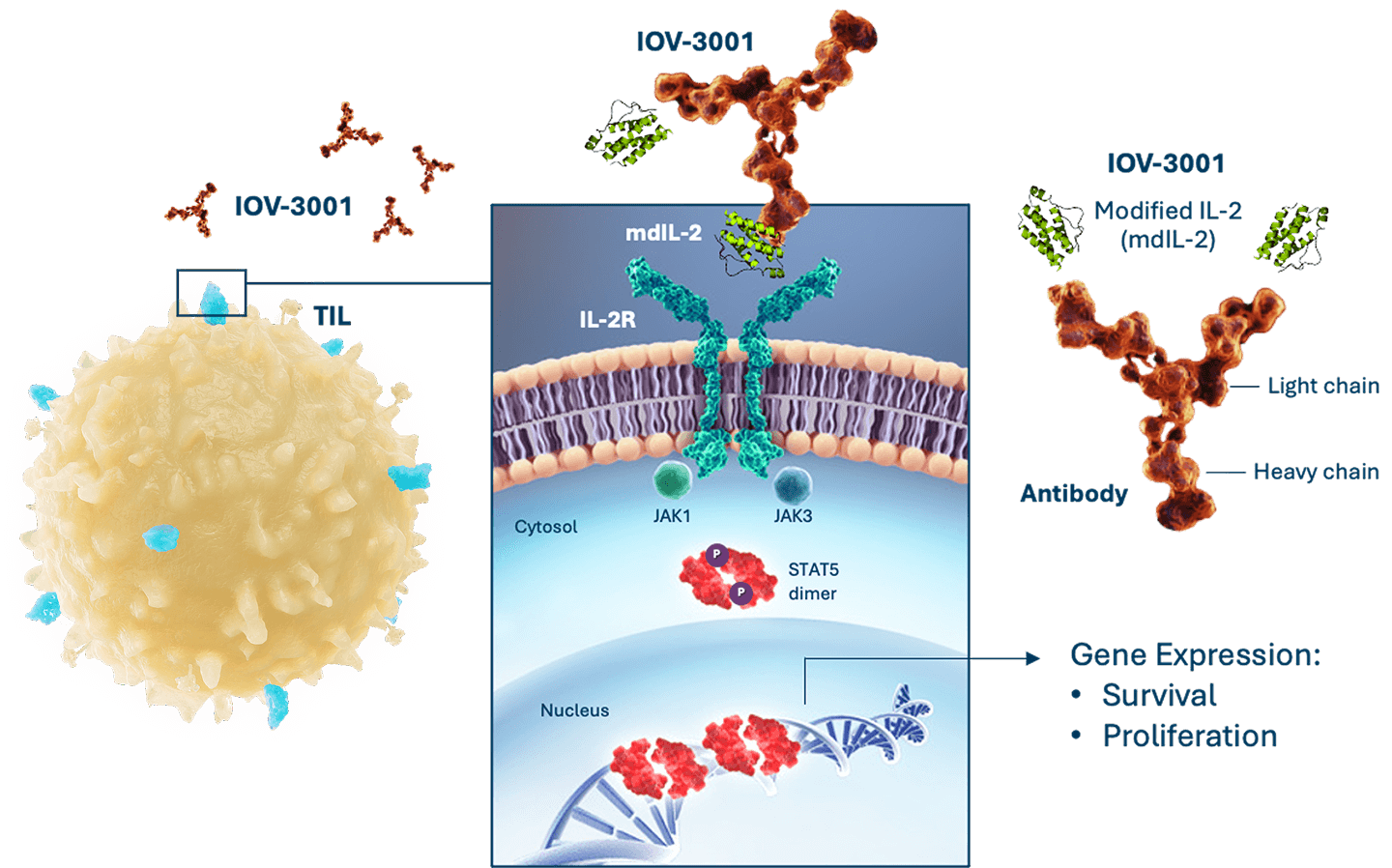
We are exploring potential improvements to the TIL treatment regimen. We are investigating IOV-3001, a second generation, modified IL-2 analog, which we licensed from Novartis Pharma AG in 2020.
IOV-3001: Next Generation IL-2 for TIL Supportive Regimen10,11
Recombinant fusion protein designed to enhance TIL survival and cellular proliferation:
- A modified copy of the coding sequence for aldesleukin (mdIL-2) is fused to a humanized monoclonal immunoglobulin (Ig)G1K antibody
- The mdIL-2 moiety of IOV-3001 binds to the IL-2-receptor (IL-2R) with subsequent phosphorylation of signal transducer and activator of transcription 5 (STAT5), resulting in enhanced performance
Preclinical data suggest IOV-3001 may have a better safety profile and require less frequent dosing compared to Proleukin
References:
1. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153-167. 2. Natarajan A, Veerapathran A, Wells A, et al. Preclinical activity and manufacturing feasibility of genetically modified PDCD-1 knockout (KO) tumor-infiltrating lymphocyte (TIL) cell therapy. Cancer Res. 2022;82(suppl 12):2746. 3. Zhang L, Morgan RA, Beane JD, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21(10):2278-2288. 4. Zhang L, Davies J, Serna C, et al. Enhanced efficacy and limited systemic cytokine exposure with membrane-anchored interleukin-12 T-cell therapy in murine tumor models. J Immunother Cancer. 2020;8(1):e000210. 5. Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827-845. 6. Zeh HJ, Hurd S, Storkus WJ, Lotze MT. Interleukin-12 promotes the proliferation and cytolytic maturation of immune effectors: implications for the immunotherapy of cancer. J Immunother Emphasis Tumor Immunol. 1993;14(2):155-161. 7. Tugues S, Burkhard SH, Ohs I, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22(2):237-246. 8. Cao X, Leonard K, Collins LI, et al. Interleukin 12 stimulates IFN-gamma-mediated inhibition of tumor-induced regulatory T-cell proliferation and enhances tumor clearance. Cancer Res. 2009;69(22):8700-8709. 9. Steding CE, Wu S, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133(2):221-238. 10. Mitra S, Leonard WJ. Biology of IL-2 and its therapeutic modulation: mechanisms and strategies. J Leukoc Biol. 2018;103(4):643-655. 11. Simpson-Abelson MR, Johnson S, Poprawski J, Blauvelt JL, Hall S, Yin H. IOV-3001, a modified interleukin-2 fusion protein, for potential use in tumor-infiltrating lymphocyte cell therapy regimens. J Clin Oncol. 2024;42(suppl 16):2552.
This site may contain information on an investigational agent(s) or investigational uses of approved agent(s) that has not been reviewed or approved by the FDA or other regulatory authorities. Iovance does not endorse or recommend any unapproved use of its products. Please refer to product prescribing information, where available.
