Proprietary Gen 2 technology
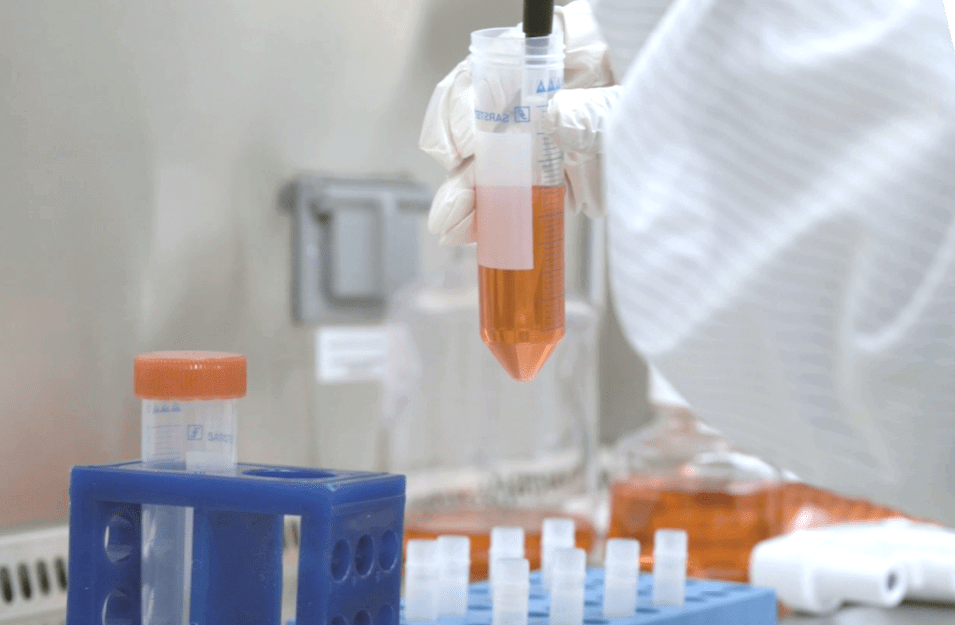
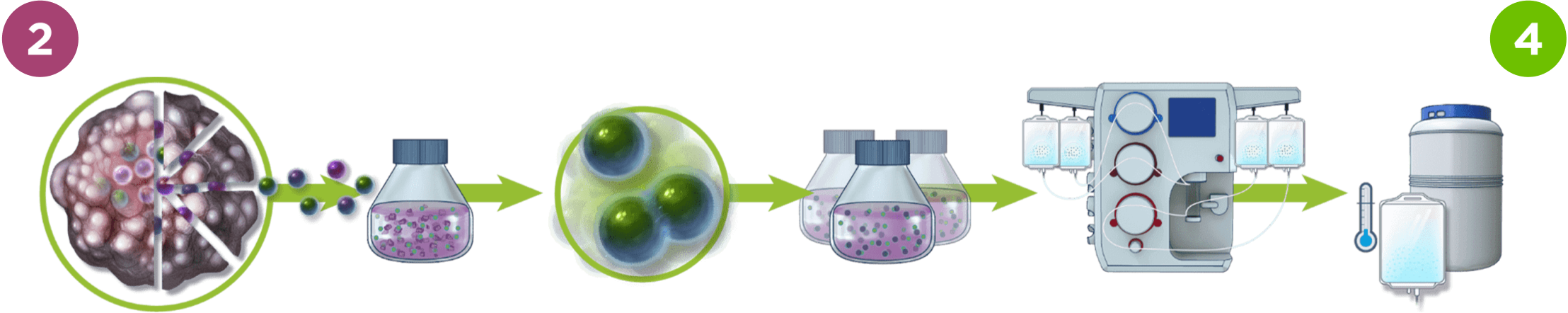
Leveraging the initial success of the NCI processes, we set out to develop centralized, scalable and proprietary processes to manufacture Iovance TIL therapies. Our proprietary Gen 2 technology includes manufacturing and logistical efficiencies aimed at further optimizing treatment, decreasing production time and streamlining distribution processes. Today, more than 700 patients have been treated with investigational TIL therapy from Iovance, with a manufacturing success rate above 90%.
Streamlined 22-day GMP manufacturing process

Our 16-day, Gen 3 process
In some of our clinical trials, we are also exploring a Gen 3 process which reduces manufacturing to 16 days. The third generation of our process is expected to reduce the wait time between initial tumor sample and infusion for patients.
GMP=good manufacturing practice; IL-2=interleukin-2; NMA-LD=nonmyeloablative lymphodepletion
This site may contain information on an investigational agent(s) or investigational uses of approved agent(s) that has not been reviewed or approved by the FDA or other regulatory authorities. Iovance does not endorse or recommend any unapproved use of its products. Please refer to product prescribing information, where available.