Key steps in the TIL therapy process
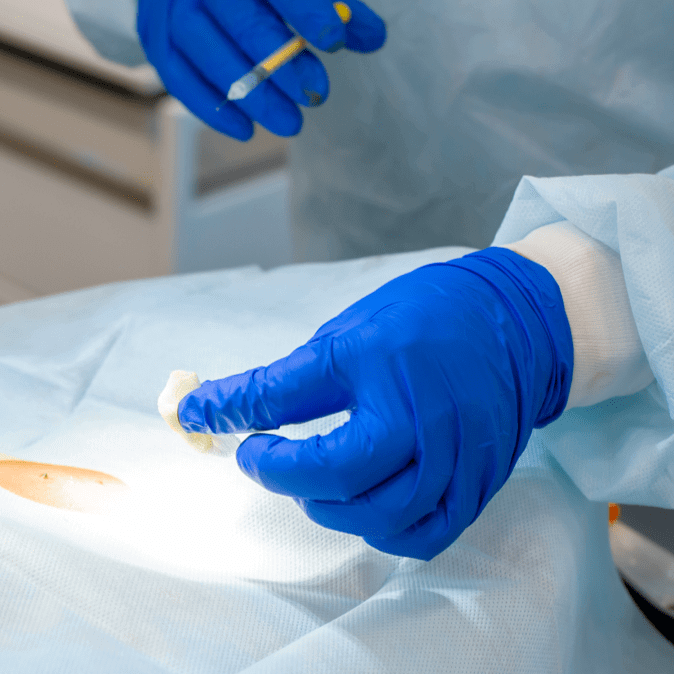
A tumor sample is removed from the patient and shipped overnight to our centralized manufacturing facility, where the TIL are isolated.

The patient-specific TIL are amplified and reinvigorated using our proprietary manufacturing process, which we refer to as Gen 2 to reflect our successful efforts to streamline TIL manufacturing from several weeks to 22 days. The TIL therapy is cryopreserved and sent back for infusion into the patient.
The patient receives a preparative regimen (lymphodepleting chemotherapy) prior to TIL infusion. The treatment regimen is completed with a short course of interleukin-2 (IL-2) to promote T-cell activity.
This site may contain information on an investigational agent(s) or investigational uses of approved agent(s) that has not been reviewed or approved by the FDA or other regulatory authorities. Iovance does not endorse or recommend any unapproved use of its products. Please refer to product prescribing information, where available.



